English
Our Research
The most crucial aspect of chemistry in the realm of science is the ability to manufacture something that does not currently exist in the world. Our goal is to create a platform of molecular design for life science and unique functional molecules with a particular emphasis on the characteristics of elements, bonds, and reaction fields. We pursue unexplored reactivity, properties, and functions through a combination of experimental science and theoretical calculation, with a focus on precise design and a thorough understanding of molecules. We also actively engage in collaborative research with various fields to expand the scope of molecular science. Some of the major themes we are exploring include the followings.
1) Development of guidelines for catalyst design and methods for functional groups introduction that utilize the characteristics of various elements, bonds, and reaction fields
2) Elucidation of molecular mechanisms that lead to high activity and selectivity
3) Creation and application of molecular probes for lipids and fatty acids in life science
4) Molecular science of bioactive compounds derived from mushrooms, so-called, “fairy chemicals”
5) Total synthesis of biologically active natural products with the potential to be used as pharmaceuticals
1) Development of guidelines for catalyst design and methods for functional groups introduction that utilize the characteristics of various elements, bonds, and reaction fields
2) Elucidation of molecular mechanisms that lead to high activity and selectivity
3) Creation and application of molecular probes for lipids and fatty acids in life science
4) Molecular science of bioactive compounds derived from mushrooms, so-called, “fairy chemicals”
5) Total synthesis of biologically active natural products with the potential to be used as pharmaceuticals
Staff
| Professor, Ph.D. | Ryo Takita | ORCID |
| Associate professor, Ph.D. | Fumihiko Yoshimura | CV |
| Assistant Professor, Ph.D. | Masaru Kondo | researchmap |
Recent Publications
2025
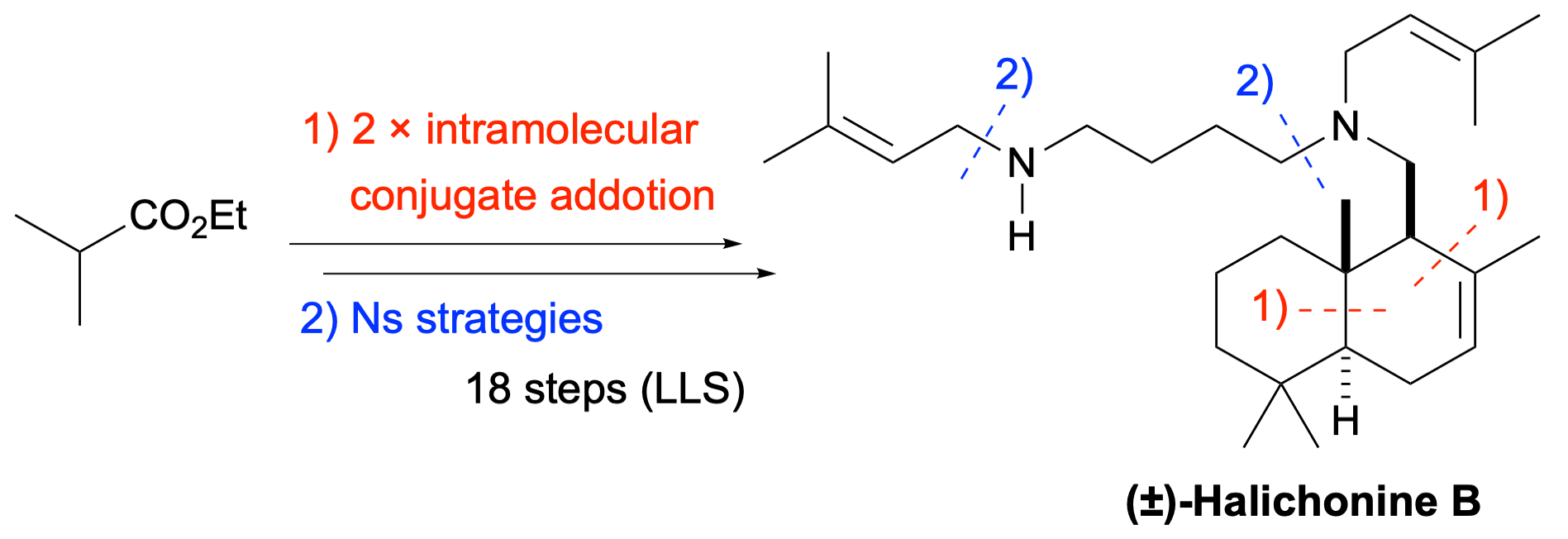
Total synthesis of (±)-halichonine B
Fumihiko Yoshimura,* Masaya Uchida, Kaori Aratame, Hitoshi Ouchi, Makoto Inai, Mitsuru Kondo, Ryo Takita, and Toshiyuki Kan
Org. Biomol. Chem. 2025, 23, 3076–3080. (DOI: 10.1039/D5OB00145E)
Formal Synthesis of Lobatamides A and C
Yuki Nakahara,Takumi Fukuda, Ryo Fujii, Masaki Kanakogi, Hitoshi Ouchi, Fumihiko Yoshimura, Ryo Takita, Toshiyuki Kan, Makoto Inai,* and Yoshitaka Hamashima*
Org. Lett. 2025, 27, 3066–3070. (DOI: 10.1021/acs.orglett.5c00739)
2024
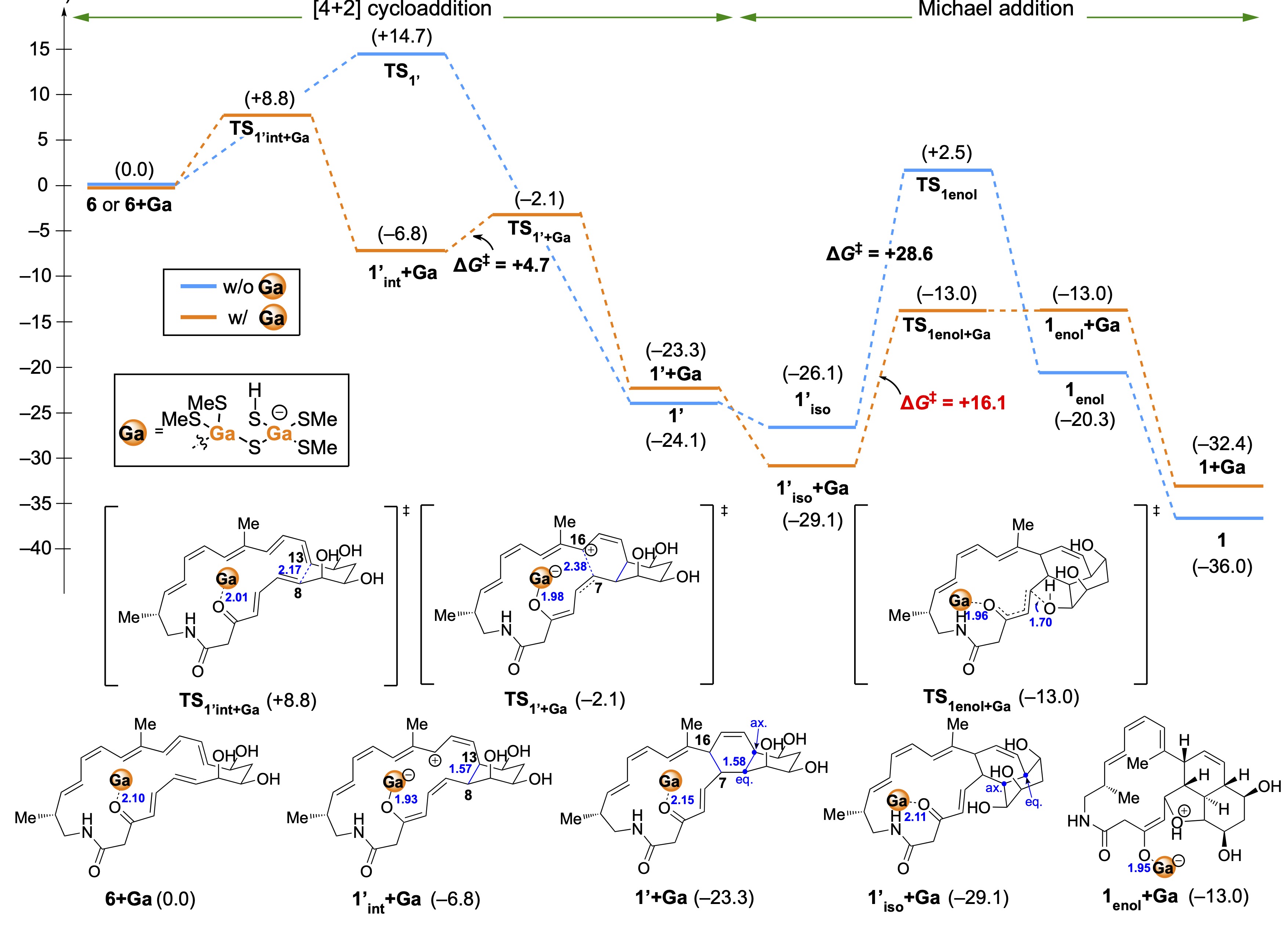
Iron-sulphur protein catalysed [4+2] cycloadditions in natural product biosynthesis
Yu Zheng,‡ Katsuyuki Sakai,‡ Kohei Watanabe,‡ Hiroshi Takagi, Yumi Sato-Shiozaki, Yuko Misumi, Yohei Miyanoiri, Genji Kurisu, Toshihiko Nogawa, Ryo Takita, Shunji Takahashi* (‡ co-first authors)
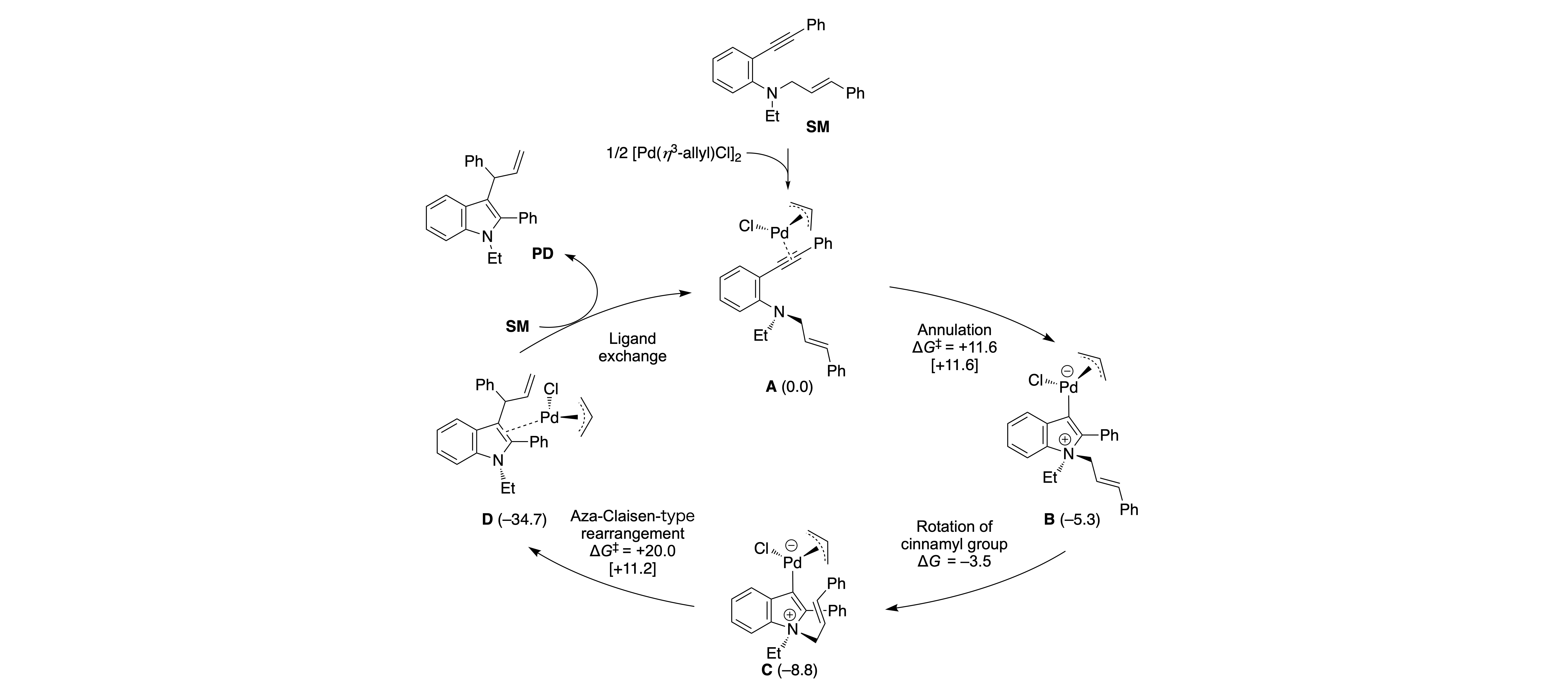
Synthesis of Branch-type 3-Allylindoles from N-Alkyl-N-cinnamyl-2-ethynylaniline Derivatives Using π-Allylpalladium Chloride Complex as a Catalyst
Kohei Watanabe*, Keita Nakano, Hayato Sato, Toshiki Yamaoka, Yasushi Yoshida, Ryo Takita, Yoshio Kasashima, Masami Sakamoto, Takashi Mino*
2023
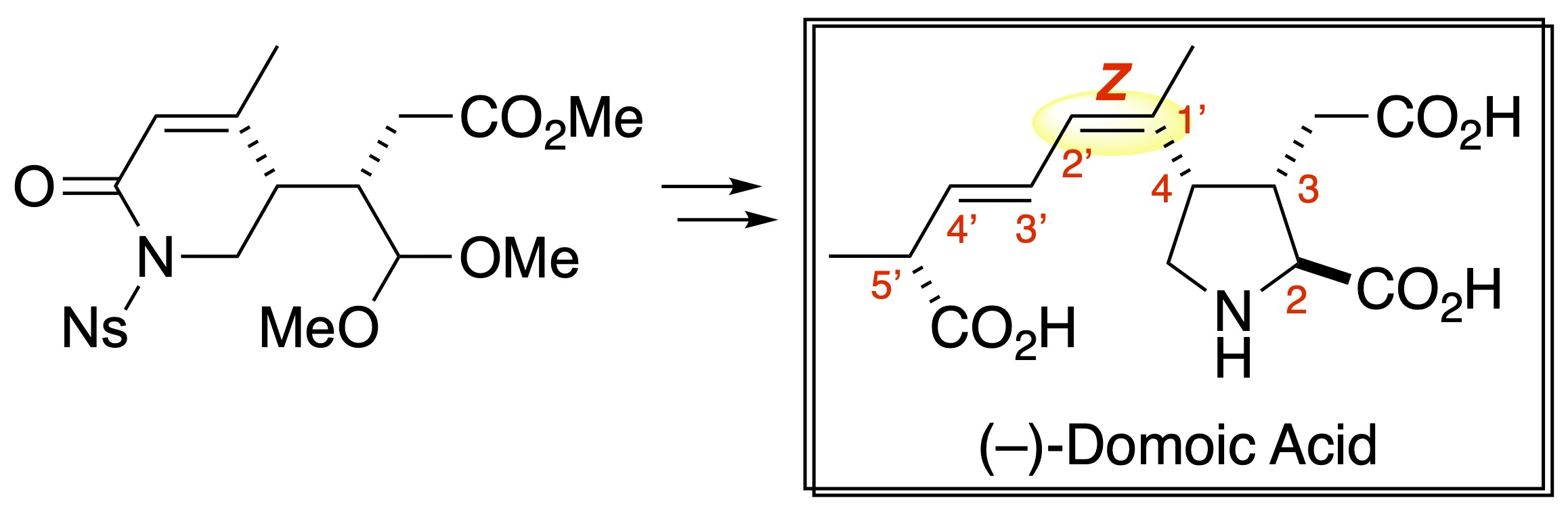
Total Synthesis of (–)-Domoic Acid, A Potent Ionotropic Glutamate Receptor Agonist and the Key Compound in Oceanic Harmful Algal Blooms
Shigeru Nishizawa, Hitoshi Ouchi,* Hiroto Suzuki, Takuma Ohnishi, Shingo Sasaki, Yu Oyagi, Masaki Kanakogi, Yoshitaka Matsumura, Shunsuke Nakagawa, Tomohiro Asakawa, Masahiro Egi, Makoto Inai, Fumihiko Yoshimura, Ryo Takita, and Toshiyuki Kan*
Org. Biomol. Chem. 2023, 21, 1653–1656. (DOI: 10.1002/d2ob02325c)
2022
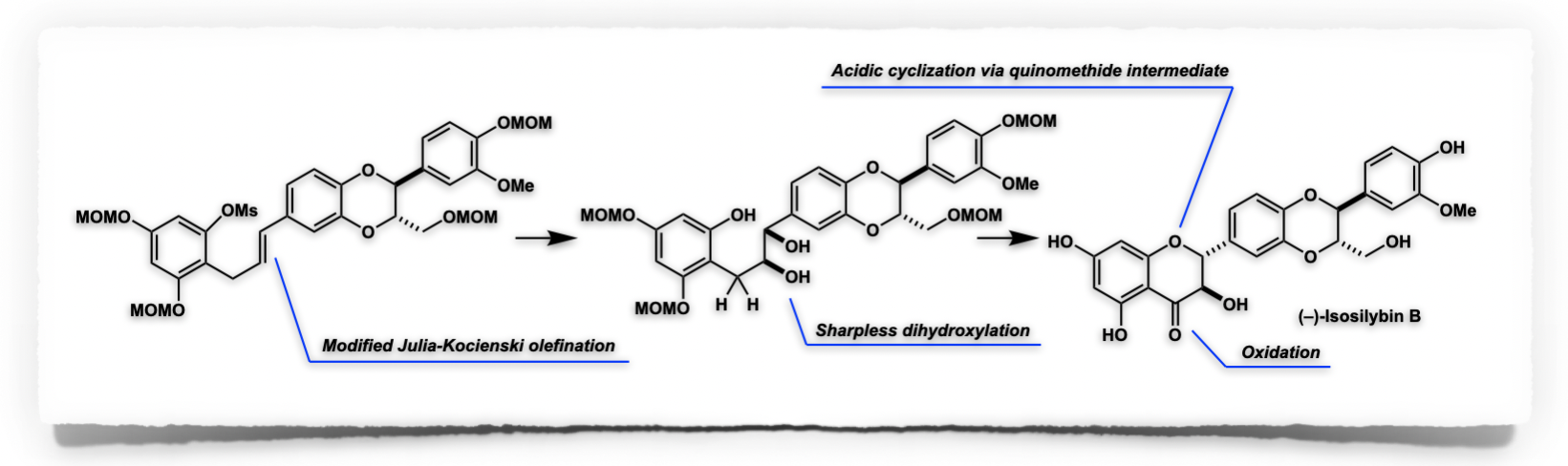
Total Synthesis of Isosilybin B
This paper is dedicated to the memory of Professor Toshiyuki Kan, who passed away on July 24th, 2021.
This paper is dedicated to the memory of Professor Toshiyuki Kan, who passed away on July 24th, 2021.
Makoto Inai,* Yoshinori Ueno, Hiroto Sagara, Hitoshi Ouchi, Fumihiko Yoshimura and Toshiyuki Kan*
Eur. J. Org. Chem. 2022, e202200653. (DOI: 10.1002/ejoc.202200653)
Controlled Tetradeuteration of Straight-Chain Fatty Acids: Synthesis, Application, and Insight into the Metabolism of Oxidized Linoleic Acid
Ayako Watanabe,‡ Kotaro Hama,*‡ Kohei Watanabe, Yuko Fujiwara, Kazuaki Yokoyama,
Shigeo Murata, and Ryo Takita* (‡ co-first authors)
Shigeo Murata, and Ryo Takita* (‡ co-first authors)
Angew. Chem. Int. Ed. 2022, 61, e202202779. (DOI: 10.1002/anie.202202779)
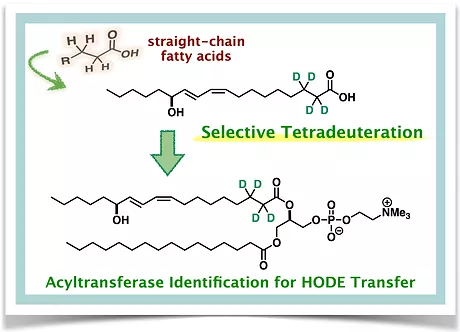
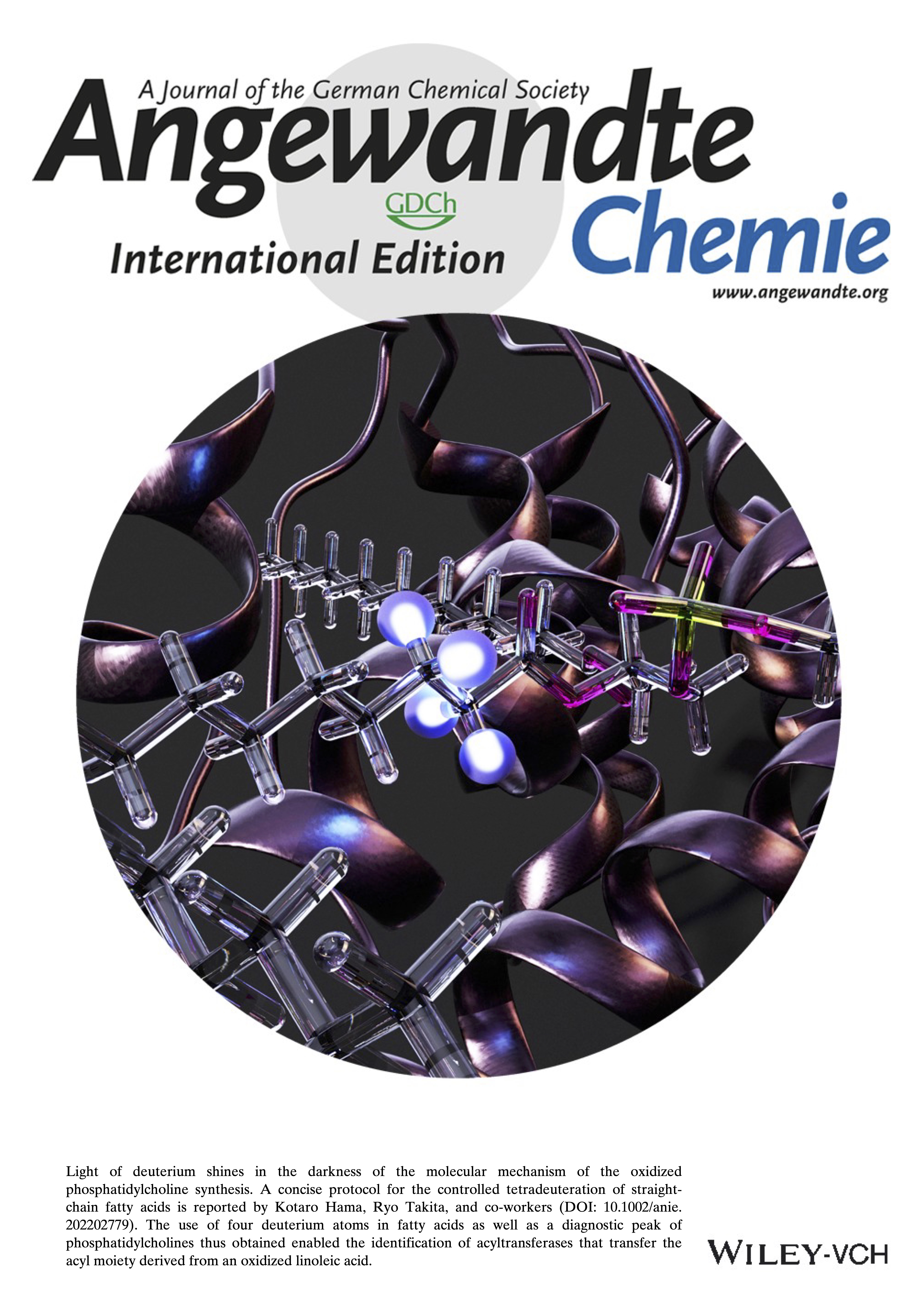
This paper has been selected to be highlighted on the front cover.
Angew. Chem. Int. Ed. 2022, 61, e202202779. (DOI:10.1002/anie.202207054)
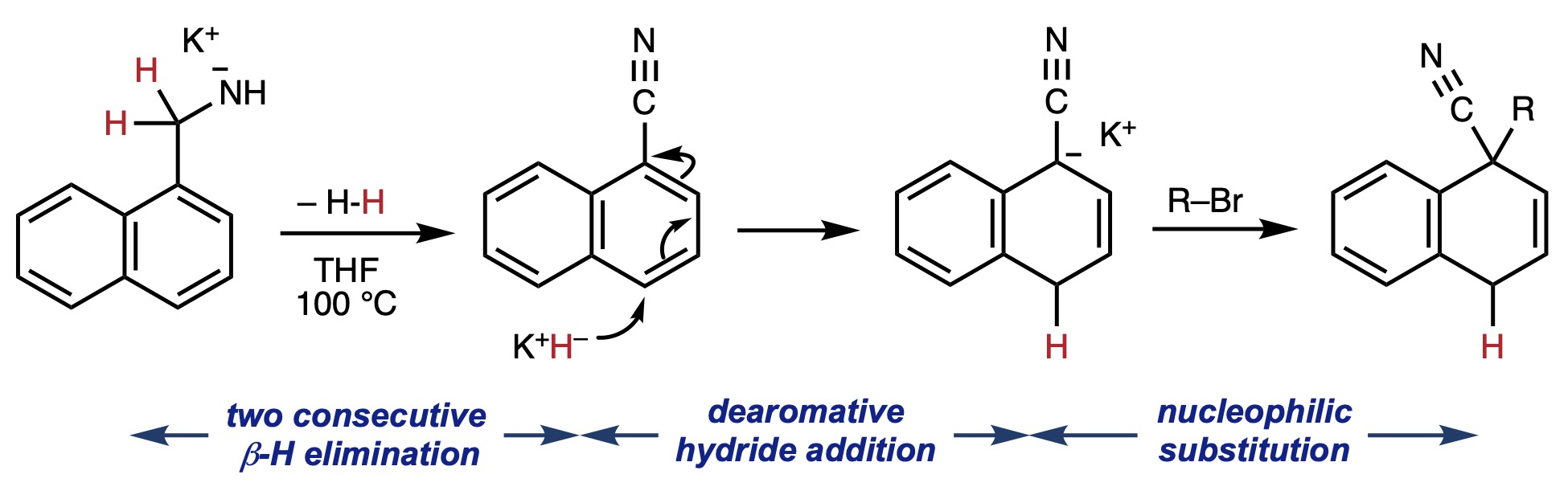
Base-induced dehydrogenative and dearomative transformation of
1-naphthylmethylamines to 1,4-dihydronaphthalene-1-carbonitriles
1-naphthylmethylamines to 1,4-dihydronaphthalene-1-carbonitriles
Yoshiya Sekiguchi, Jia Hao Pang, Jia Sheng Ng, Jiahua Chen, Kohei Watanabe, Ryo Takita* and Shunsuke Chiba*
JACS Au 2022, 2, 2758-2764. (DOI: 10.1021/jacsau.2c00487)
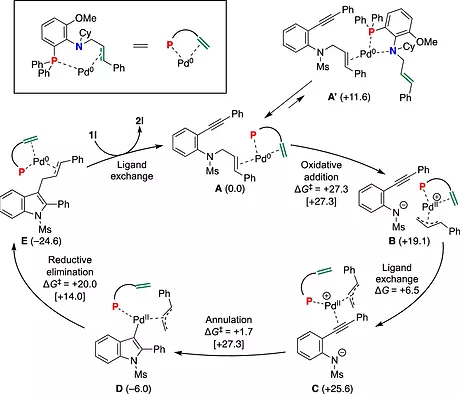
Synthesis of 3-Allylindoles via Annulation of N-Allyl-2-ethynylaniline Derivatives Using a P,Olefin Type Ligand/Pd(0) Catalyst
Takashi Mino,* Toshiki Yamaoka, Kohei Watanabe,* Chihiro Masuda, Shohei Kasano, Yasushi Yoshida, Ryo Takita, Yoshio Kasashima, and Masami Sakamoto
J. Org. Chem. 2022, 87, 7365-7377. (DOI: 10.1021/acs.joc.2c00588)
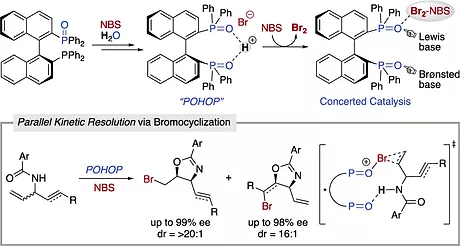
Mechanistic Details of Asymmetric Bromocyclization with BINAP Monoxide: Identification of Chiral Proton-Bridged Bisphosphine Oxide Complex and Its Application to Parallel Kinetic Resolution
Kenji Yamashita, Ryo Hirokawa, Mamoru Ichikawa, Tatsunari Hisanaga, Yoshihiro Nagao, Ryo Takita,
Kohei Watanabe, Yuji Kawato, and Yoshitaka Hamashima
Kohei Watanabe, Yuji Kawato, and Yoshitaka Hamashima
J. Am. Chem. Soc. 2022, 144, 3913-3924. (DOI: 10.1021/jacs.1c11816)
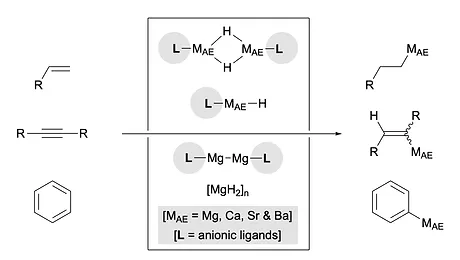
Generation of organo-alkaline earth metal complexes from non-polar unsaturated molecules and their synthetic applications
Kohei Watanabe‡, Jia Hao Pang‡, Ryo Takita,* and Shunsuke Chiba* (‡ co-first authors)
Chem. Sci. 2022, 13, 27-38. (perspective) (DOI: 10.1039/D1SC05724C)
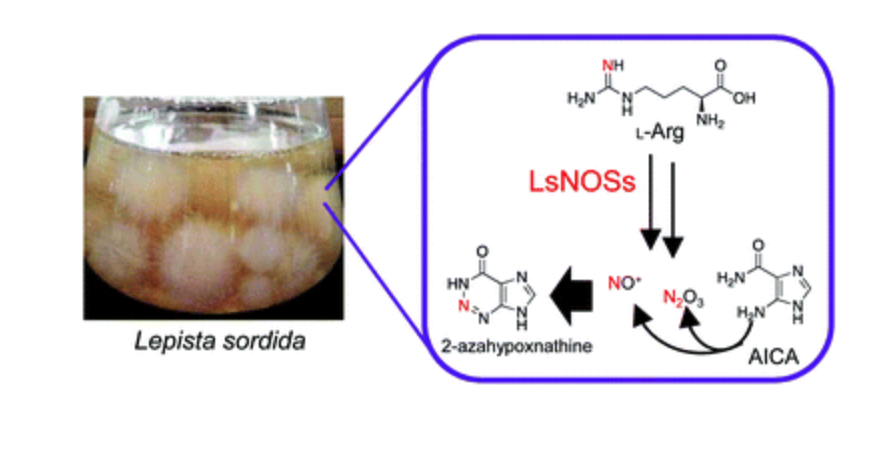
1,2,3-Triazine formation mechanism of the fairy chemical 2-azahypoxanthine in the fairy ring-forming fungus Lepista sordida
Akinobu Ito,‡ Jae-Hoon Choi,‡ Waki Yokoyama-Maruyama, Mihaya Kotajima, Jing Wu, Tomohiro Suzuki,
Yurika Terashima, Hyogo Suzuki, Hirofumi Hirai, David C. Nelson, Yuta Tsunematsu, Kenji Watanabe,
Tomohiro Asakawa, Hitoshi Ouchi, Makoto Inai, Hideo Dohra, Hirokazu Kawagishi* (‡ co-first authors)
Yurika Terashima, Hyogo Suzuki, Hirofumi Hirai, David C. Nelson, Yuta Tsunematsu, Kenji Watanabe,
Tomohiro Asakawa, Hitoshi Ouchi, Makoto Inai, Hideo Dohra, Hirokazu Kawagishi* (‡ co-first authors)
Org. Biomol. Chem. 2022, 2636–2642. (DOI: 10.1039/D2OB00328G)